Patient Portal
Disclaimer: Chondrograft™ is not approved yet for marketing in the USA. This information should be considered informational only.
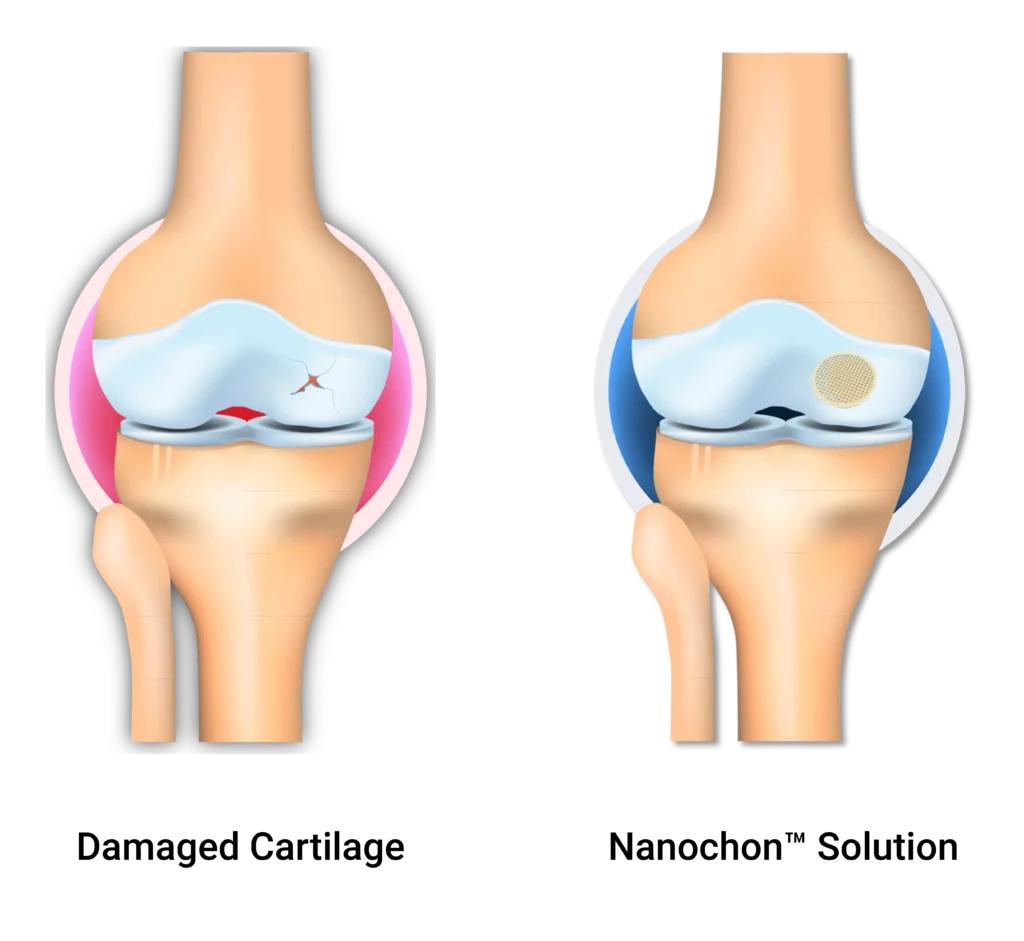
Are you having knee pain because of an articular cartilage lesion?
Non-Operative Treatment
Conservative or non-operative care is offered to patients before surgery. The treatment may vary across doctors and may include one or all of the following:
- Oral analgesics
- Anti-inflammatory medication (e.g., ibuprofen, naproxen)
- Physical therapy
- Physician-directed at-home exercise program
- Bracing
- Activity modification
Patients who do not respond to or are unsatisfied with non-operative treatment(s) may seek surgical treatment.
Surgery
Surgery may be considered to treat the cartilage defect. There are several different surgical options, and these should be discussed with your doctor.
FREQUENTLY ASKED QUESTIONS
The 3D printed Chondrograft™ implant is primarily composed of Nylon 12 which is insoluble and has a long-standing and established history of use in medical devices. The secondary component, PVA, is soluble and provides increased surface roughness and is a common additive and encapsulation material in the pharmaceutical industry. All implants are cylindrical in shape.
What are the Chondrograft materials?
Chondrograft™ is designed to provide a scaffold for healing of lost or damaged cartilage on the articular surfaces of the knee. During this time it is expected that the regenerated tissue will fill the defect and potentially integrate with the implant through a mixture of cartilage tissue composed of fibrocartilage, fibrous connective tissue, and hyaline-like cartilage.
What is the Chondrograft mechanism of action?
The minimally invasive mini-open or arthroscopic procedure can be performed in a surgery center or hospital setting.
Where does the procedure take place?
Patients can return home the same day following the procedure.
When can I go home after the procedure?
Each patient may have a different recovery outcome. Your doctor will determine the best rehabilitation program for you. Chondrograft™ is designed to provide mechanical support that may allow patients to walk on the same day as surgery and resume limited activity immediately. Full recovery may take four to six months.
What does recovery look like?
Chondrograft™ is not approved yet for marketing in the USA. Nanochon™ is planning to conduct a North American clinical study.
